
Moreover, helium is lighter than air, but hydrogen is slightly heavier than air. Compared to hydrogen, helium is an inert gas. Helium has a fully filled s orbital (1s2), but in hydrogen, there is only one electron (1s1), so it is unstable. The key difference between hydrogen and helium is that hydrogen is a diatomic gas, while helium is a monatomic gas. Hydrogen and helium are the smallest elements and these are the first two elements in the periodic table. What is the Difference Between Hydrogen and Helium? Furthermore, it is useful for magnetic resonance imaging (MRI), pressurizing liquid fuel rockets, as a cooling medium for nuclear reactors, etc.
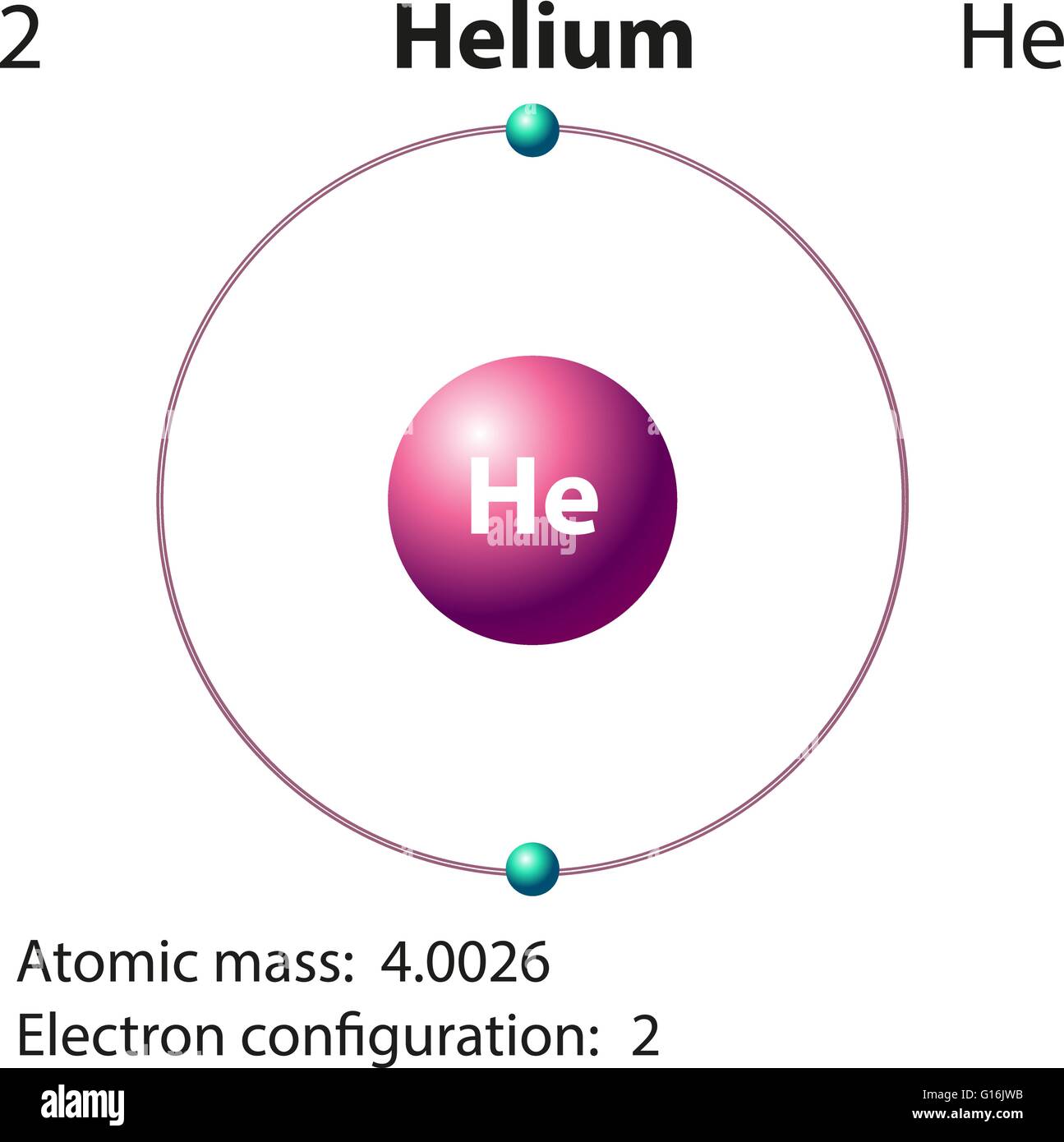
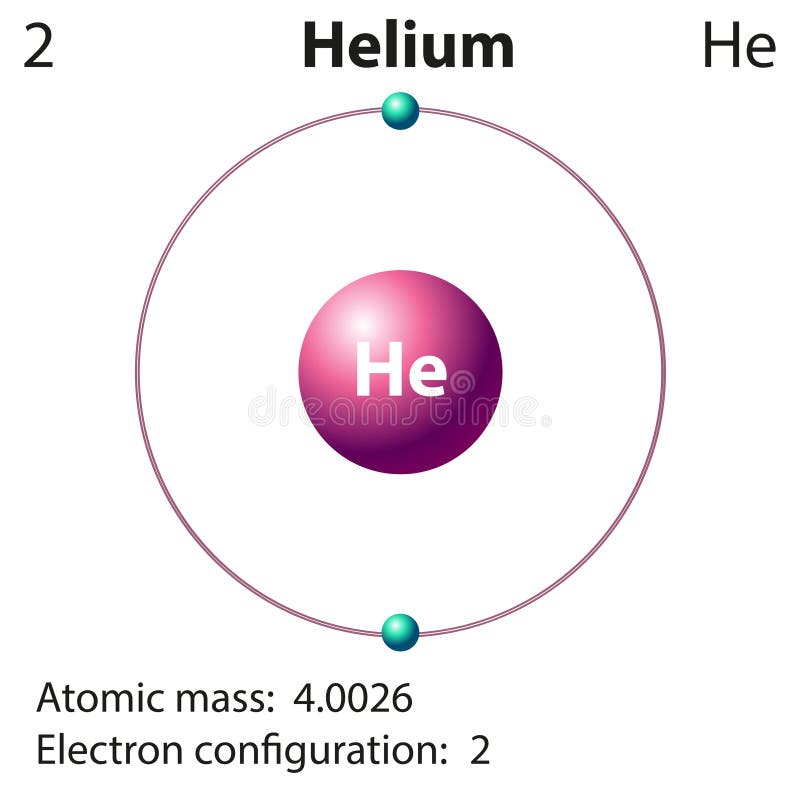
Helium is lighter than air, so it is used to fill balloons, airships, etc. Moreover, this gas has seven isotopes, among those only He-3 and He-4 are stable. The melting point (0.95 K) and the boiling point (4.22 K) of helium are considered as the lowest values among other elements. It also has a low boiling point, low density, low solubility and high thermal conductivity. Helium is a light, colourless, odourless gas like hydrogen. The molecular weight of helium is 4 g mol-1. S orbital can only accommodate two electrons, so in helium s orbital is fully filled, making helium an inert gas. It has two electrons therefore, the electron configuration is 1s2. Helium (He) is the second element in the periodic table, and it is in group 18 (Nobel gas). Moreover, liquid hydrogen is important as a fuel in rockets and vehicles. Hydrogen is useful in chemical industries such as ammonia production in the Haber process. H2 is in the zero oxidation state therefore, it can act as a reducing agent to reduce metal oxides, or chlorides and release metals. However, in high temperatures, it can react fast. Under normal room temperature, it is not very reactive. Furthermore, it is an extremely flammable gas and it burns with a pale blue flame. Hydrogen exists as a diatomic molecule (H2) in the gas phase, and it is a colourless, odourless gas. Protium is the most abundant among three, having about 99% relative abundance. Because of this ability, hydrogen is present in a large number of molecules, and it is a highly abundant element in the earth.įurthermore, hydrogen has three isotopes named as protium-1H (no neutrons), deuterium-2H (one neutron), and tritium– 3H (two neutrons). Hydrogen can take up an electron to form a negatively charged ion, or can easily donate an electron to produce a positively charged proton or share an electron to make covalent bonds. We can categorize it under group 1 and period 1 in the periodic table because of its electron configuration: 1s1. Hydrogen is the first and the smallest element in the periodic table, which is denoted as H. Side by Side Comparison – Hydrogen vs Helium in Tabular Form Hydrogen only has one electron, and it can achieve the electron configuration of Helium by obtaining another one. They are very simple elements having electrons filled to 1s orbital only. Both are gases and have a high abundance in the universe.
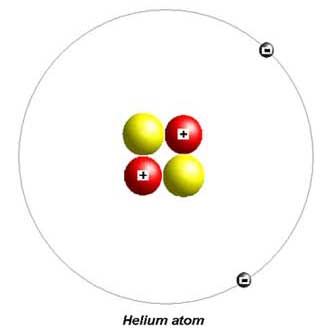
Hydrogen and helium are the first two elements in the periodic table.


 0 kommentar(er)
0 kommentar(er)
